Feds push animal testing alternatives; Wisconsin laboratory leads the way
Feds push animal testing alternatives
The federal government is pushing alternatives to animal testing. A Wisconsin-based laboratory is leading the way.
MILWAUKEE - The U.S. Food and Drug Administration has "groundbreaking" plans to phase out animal testing for certain new drugs in the next three to five years. The agency said it plans to incentivize the very kind of research already underway at a biotech startup in Madison that is developing a treatment for Alzheimer's.
Drug research without animals
The backstory:
Thirty miles west of Madison, Ridglan Farms sells beagle puppies for use in laboratory experiments. It is also the subject of an ongoing animal cruelty investigation, and several staff members face disciplinary investigations as activists call for the state to confiscate thousands of would-be lab dogs.
FREE DOWNLOAD: Get breaking news alerts in the FOX LOCAL Mobile app for iOS or Android
Meanwhile, researchers at Madison-based Stem Pharm are working on a new approach to drug development that could reduce or replace the need for animal experiments altogether.
Stem Pharm's Chief Operating Officer Connie Lebakken gave the FOX6 Investigators a tour of the laboratory in May that included a cryogenic tank that stores billions of living cells needed for research.

Researchers at Madison-based Stem Pharm are working on a new approach to drug development that could reduce or replace the need for animal experiments
"These racks are filled with different cell types," Lebakken said as she removed the tank's lid, allowing a whoosh of nitrogen gas to escape.
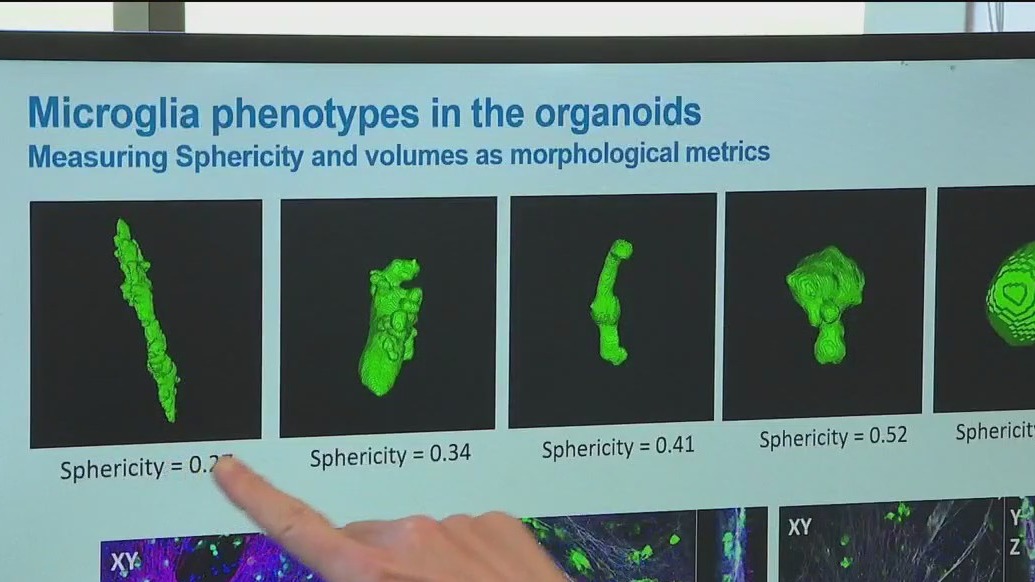
The scientists at Stem Pharm are using adult stem cells to create brain cells that are added to a living micro-environment, complete with "microglia," the brain's resident immune cells. The result is a three-dimensional organoid.
"It’s a complex model of what’s happening in the brain," she said.
Factory reset
Dig deeper:
The microscopic, organ-like tissues are created from real human skin and blood cells that have been reprogrammed into pluripotent stem cells, which are master cells that can then be purposely differentiated into specific cell types.
"It’s very much like a factory reset," Lebakken said.
At Stem Pharm, they are turning those stem cells into neurons – the centerpiece of their neural organoids.

Researchers at Madison-based Stem Pharm are working on a new approach to drug development that could reduce or replace the need for animal experiments
"The idea is you’ve now captured complex human biology in the laboratory that should give you better clinical predictability," said Steven Visuri, Stem Pharm CEO.
Over the past 10 years, Stem Pharm has secured nearly $6 million in federal funding for drug development and research. First, they created a healthy neural organoid in the lab. Then, they subjected the healthy model to conditions that mimic the inflammation seen in a person with Alzheimer's. Next, they hope to test experimental compounds on the inflamed organoid, hoping to identify a new treatment.
Nowhere in the process do they involve any animals.
Discover better drugs
Why you should care:
Visuri said the organoid models are not just alternatives to animal testing. They are superior to it.
"This will discover better drugs," he said. "You are using a more physiologically relevant model. A better representation of the brain's biology in the laboratory which should help you better predict drugs that will work in real patients when you get to that point."
Amy Van Aartsen agrees. She is a self-described beagle lover and co-founder of the Marty Project, which promotes the replacement of dogs in lab testing with new technologies.

The Marty Project
While she does not speak for the University of Wisconsin – or any other laboratory for that matter – she does work on the UW-Madison campus where she turns adult stem cells into specialized heart cells.
"As they become cardiomyocytes in nature they will essentially spontaneously contract in the dish," she said, "which looks like beating heart cells."
Those cells can then be added to an organ chip which emulates a living organ.
"So the cells I am working to help develop, those then can be used to mimic the heart," she said.
Reduce, replace, refine
Big picture view:
Organoids, organ chips, computational models and artificial intelligence are driving a fundamental shift in how pre-clinical research is conducted in the United States.
Sometimes referred to as "non-animal methods," "novel alternative methods" or "new approach methodologies," so-called "NAMs" are showing increased promise for reducing, replacing and refining traditional lab testing on mice, rabbits, pigs and, sometimes, more controversial research subjects, like the dogs bred for the lab at Ridglan Farms.
"I see these dogs in their cages and video of everything at Ridglan and I see my Maggie," Van Aartsen said. "I see that could have been her and just how awful it is to see that."

Beyond the ethical concerns, Visuri said animal models are simply unreliable, since most drugs that succeed in animals are never approved for human use.
"They’re just poor predictors of clinical outcomes," he said, "so there’s no reason we should be using these animals if it’s a flip of the coin whether it’s going to work or not."
That can be both time-consuming and expensive for drug companies.
"If you’re going to fail, you want to fail quickly," Lebakken said. "And so I think that these models will help us fail faster."
‘Science is not there yet’
The other side:
A recent survey found 85% of Americans – both Democrats and Republicans – support phasing out animal testing in favor of new methods. However, that survey was conducted by a group that opposes animal testing.
Visuri said animals are still needed for now, and he's not sure how long it will be until they can be eliminated from drug research altogether.
"I think the biggest impediment is the way we’ve always done it," he said.

Researchers at Madison-based Stem Pharm are working on a new approach to drug development that could reduce or replace the need for animal experiments
"Can we use organ chips for every single research question?" asked Naomi Charalambakis with Americans for Medical Progress. "No. The answer is no. The science is not there yet."
Charalambakis acknowledges that new methods have shown promise in the area of drug toxicology testing.
"But in areas like neuroscience and behavioral studies, like studying mental health, you can't really replicate that information with just a chip in isolation. You still need that living system," she said.
'Quite frankly, it's bulls***'
What they're saying:
Craig Reinemeyer agrees.
"Some of the organs are so incredibly complicated they will never be replicated in vitro," said Reinemeyer, founder of East Tennessee Clinical Research and a top customer of Ridglan Farms.
"I think the notion of being able to do everything that I do in a petri dish," he said, "quite frankly, it's bull****, okay?"
"There's a very strong bias in the scientific community towards animal studies," said Delci Winders, director of the Animal Law and Policy Institute.
"We do have the technology to replace the animals," she said. And Winders believes the federal government is finally moving in the right direction.

In April, the FDA announced a new plan to phase out animal testing for experimental drugs that use monoclonal antibodies to target disease. According to the FDA's 11-page "roadmap" to reducing animal testing, the agency intends to make animal testing the "exception" rather than the norm for preclinical toxicity and safety testing within 3 to 5 years.
SIGN UP TODAY: Get daily headlines, breaking news emails from FOX6 News
Days after the FDA announcement, the National Institutes of Health promised to prioritize human-based technologies and to spend $400-million over ten years to develop and validate non-animal approaches.
"I think the government getting behind this in such a vocal and explicit and unequivocal way, and putting money behind it is going to be game-changing," she said.
Change Doesn't Happen Overnight
What's next:
Still, Visuri said change does not happen overnight.
"I think at some point they will be phased out, but I think that’s a long way from now."
For now, the researchers at Stem Pharm are focused on one thing - making life better for people.

Researchers at Madison-based Stem Pharm are working on a new approach to drug development that could reduce or replace the need for animal experiments
"Not only increase lifespan, but the quality of life. My mom has Alzheimer's disease," her voice suddenly choked. "I mean, so the ultimate goal is to help people, right? Lead better lives."
A potential boon for mankind – and man's best friend.
Ridglan Farms is facing a series of ongoing investigations and complaints, including a criminal review for possible animal cruelty. Eight staff members have pending state disciplinary investigations before the Veterinary Examining Board or Department of Agriculture, Trade and Consumer Protection.
Meanwhile, Ridglan Farms is suing Dane4Dogs, the organization that prompted the criminal review. Ridglan accuses Dane4Dogs of trying to interfere with contractual relationships with customers. In a motion to dismiss, attorneys for the activist group say Dane4Dogs engaged in protected First Amendment activities including protests and filing complaints with government agencies.
The Source: The information in this post was collected and produced by FOX6 News.

