COVID-19 vaccine: UW Health expert urges trust in FDA's process

UW Health expert urges trust in FDA’s process
Staff at UW Health are already preparing for a COVID-19 vaccine, practicing vaccinations this week in anticipation of FDA emergency use authorization.
MILWAUKEE - The U.S. Food and Drug Administration (FDA) is expected to approve Pfizer's COVID-19 vaccine for emergency use during a meeting on Thursday, Dec. 10.
The FDA committee will review a 53-page Pfizer document explaining the process, study and efficacy results of its proposed vaccine.
A UW Health vaccine expert said to think of this as Pfizer's final thesis, which could green-light immunization efforts as soon as next week.
Staff at UW Health are already preparing for a COVID-19 vaccine, practicing vaccinations this week in anticipation of FDA emergency use authorization.
"This is sort of the final paper that companies submit," said Dr. James Conway, medical director of immunization programs at UW Health.
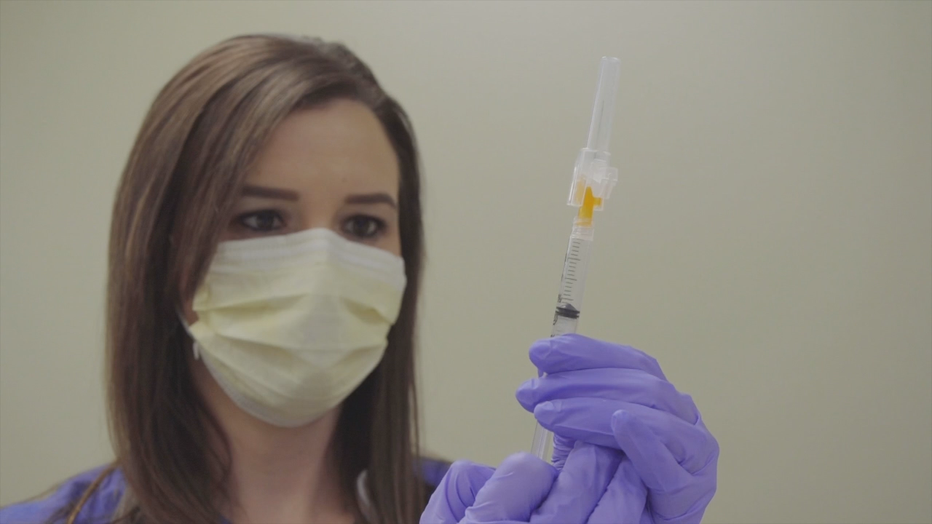
UW Health staff practice administering a COVID-19 vaccine
Conway said most vaccines develop over five to 10 years. Pfizer and BioNTech have cut that timeframe to nine months under Operation Warp Speed. It is a change that Conway hopes is the new normal and shouldn't cause concern.
"It was speeding things up, but it wasn't cutting corners. It was basically just getting every member of FDA to direct all their attention to how do we do this well and how do we review this all along the way," Conway said.
The committee will review Pfizer's study, including a look at side effects to the vaccine that must be administered in two doses, three weeks apart.

"The second dose seems to even have more side effects, some headache, some achiness, and quite a few people feel kinda fatigued for one to three days afterward. And about half the people actually do have a little bit of a low-grade fever," said Conway.
However, Conway notes that vaccines are meant to protect against severe disease -- not prevent the disease altogether.
Should emergency approval happen Thursday, it would not be long before immunizations begin across the country.
"The vaccine's going to be starting to move out to hubs across Wisconsin and across the country by early next week, so we're hopeful that we'll be able to start immunizing people by the end of next week," said Conway.
FREE DOWNLOAD: Get breaking news alerts in the FOX6 News app for iOS or Android.
The FDA's Vaccines and Related Biological Products Advisory Committee meeting is expected to last all day.
A U.S. Centers for Disease Control and Prevention committee will also meet this weekend to officially determine who gest the first doses of vaccine.
Featured
Wisconsin could play key role in COVID-19 vaccine distribution
Once allowed, Pfizer plans to ship vaccines largely from two hubs -- one in Pleasant Prairie, one in Kalamazoo, Michigan.
Featured
DHS campaign aims to boost flu vaccine rates in communities of color
Wisconsin’s Department of Health Services (DHS) launched on Wednesday, Dec. 9 the “Be an InFLUencer” education and awareness campaign.



