White House coronavirus task force: Vaccine implementation won’t reduce spread of disease until late spring
LOS ANGELES - As the United States awaits approval from federal regulators for emergency use of a COVID-19 vaccine, The White House coronavirus task force is warning that current vaccination plans won’t necessarily have a dramatic impact on the spread of the disease until at least late spring of 2021.
“The current vaccine implementation will not substantially reduce viral spread, hospitalizations, or fatalities until the 100 million Americans with comorbidities can be fully immunized, which will take until the late spring,“ the report read. "Behavioral change and aggressive mitigation policies are the only widespread prevention tools that we have to address this winter surge."
The report which has been distributed weekly detailing current COVID-19 trends for each state added that while imminent arrival of vaccines provides hope," it will still take months for people to see immediate effects.
RELATED: More than 1 million confirmed US coronavirus cases were reported in the first 5 days of December
"The testing expansion across November has been a remarkable achievement and will be needed to effectively address this winter surge; continue efforts to expand capacity and make testing easily accessible to all populations, particularly in communities that are undertesting. " the report said. "Mitigation efforts must increase, including the implementation of key state and local policies with an additional focus on uniform behavioral change including masking, physical distancing, hand hygiene, no indoor gatherings outside of immediate households, and aggressive testing to find the asymptomatic individuals responsible for the majority of infectious spread."
The task force issued another eerie warning in a report issued Nov. 29, saying the U.S. is currently in “a very dangerous place” in the pandemic due to a heavy surge in COVID-19 cases throughout the country.
“The COVID risk to all Americans is at a historic high," said the report from the task force, which was released by multiple state officials detailing current COVID-19 trends around the country.
In the first five days of December alone, the U.S. added more than 1 million coronavirus cases as the country continues to hit record averages for virus cases and deaths.
Cases per day have eclipsed 200,000 on average for the first time on record, with the crisis likely to get worse because of the fallout from gatherings at Thanksgiving, Christmas and New Year’s as the nation inches towards 300,000 coronavirus deaths.
A record of more than 107,000 people were in the hospital in the U.S. with COVID-19 as of Thursday, according to the COVID Tracking Project. More than 290,000 Americans have died of the virus.
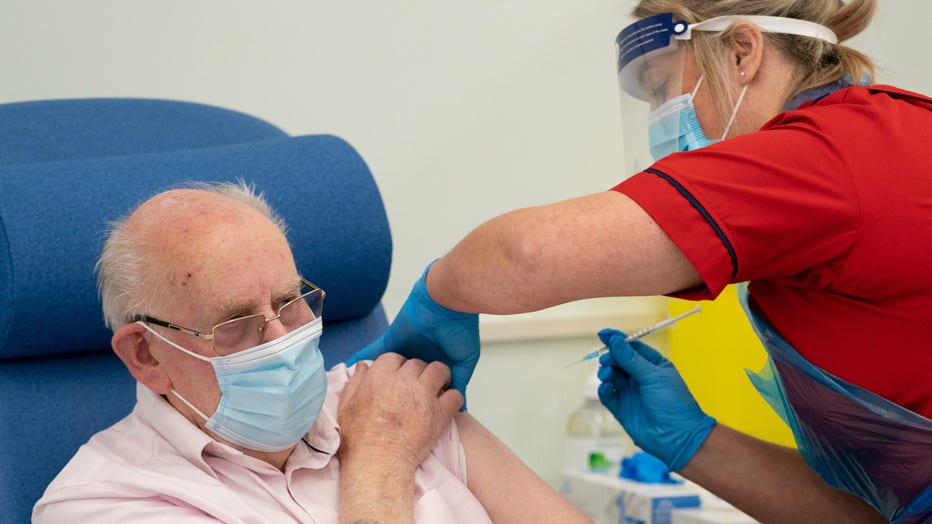
General manager of Covid Recovery, Becky Board, administers the first Pfizer-BioNTech COVID-19 vaccine in London to patient George Dyer, 90, at Croydon University Hospital, at the start of the largest ever immunisation programme in the UK's history o (Photo by Dan Charity - Pool/Getty Images)
As the country remains desperate for an end to a seemingly endless and disastrous pandemic, White House chief of staff Mark Meadows on Friday pressed Food and Drug Administration chief Stephen Hahn to grant an emergency use authorization for Pfizer's coronavirus vaccine by the end of the day or face possible firing, two administration officials said.
President Donald Trump’s frustration with the FDA has been mounting, particularly as other countries have beaten the U.S. in issuing emergency approvals for the vaccine.
In a separate move Friday, Trump administration officials announced the purchase of 100 million additional doses of a different upcoming vaccine from drugmaker Moderna. That's on top of a previous purchase of 100 million doses. FDA's expert advisory panel is scheduled to review that vaccine next Thursday, with a decision soon thereafter.
The Associated Press contributed to this story.


